Quality
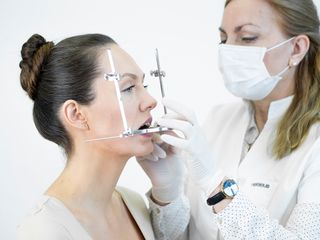
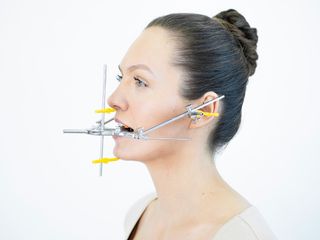
The Gerber Medical Diagnosis System comprises the following components: Gerber Facebow – DED® (Dynamic Esthetic Diagnostics), Gerber Registration Instruments®, and Gerber Condylator®. The Gerber Medical Diagnosis System provides a foundation for dental rehabilitation, enabling the reconstruction of damaged or missing dentition in an anatomically and physiologically correct relationship to the temporomandibular joint (TMJ).
The registration method can be applied in diagnosing occlusal disharmonies related to dentition, periodontal disease, and/or temporomandibular joint disorders. Values obtained through instrumental occlusal analysis in relation to the TMJs are utilized in the fabrication of orthodontic and prosthodontic appliances, including crowns, bridges, occlusal splints, and both fixed and removable restorations.
The Gerber Medical Diagnosis System allows precise control of the ortho-angulation of working and balancing facets in natural and artificial dentition. It enables an anato-mechanical simulation of condylar paths from centric relation to the most retruded and most protrusive mandibular positions, providing full three-dimensional control of Bennett movement and Immediate Side Shift (ISS).


The Gerber Condylator’s medical products meet certified safety standards and legal requirements, and are CE marked as Class I Medical Devices.
Declaration of Conformity of: Gerber Facebow – DED®, Gerber Condylator®, Gerber Registration Instruments® are available by email upon request. Please contact us ito receive these documents.
The Gerber Condylator GmbH has designated Manufaktura Dentystyczna – Paulina Owoc as the European Authorized Representative under the Medical Device Regulation. For questions or complaints, contact the European Authorized Representative at ear@condylator.com or the Gerber Condylator directly at gerber@condylator.com.
Note: Regulatory approval for products varies by country. For more information or details on local distributors and agents, contact our Sales and Marketing team at gerber@condylator.com.
All components of the Gerber Medical Diagnosis System are protected worldwide by trademarks and patents.
The Gerber Condylator® and Gerber Facebow–DED® can be sent in for service. Spare parts are not distributed separately. Repairs are accepted only after a quotation is provided. Quotations are prepared based on a set of photographs showing the equipment’s condition. All costs related to repair, including shipping to and from our facility, are the client’s responsibility.
Instruction for Use / Product Manuals
Please note: Regulatory approval for products varies by country. For further information and details of local distributors and agents, contact our Sales and Marketing team at gerber@condylator.com.
IFU – Library
